Millions of fogeys be afflicted by untreated ache for infinite diseases and diseases. In certainty, on account that 1986, the World Health Organization (WHO) has noticed that insufficient remedy of cancer and non-cancer ache is a primary public smartly being worry.
Therefore, contained in the face of this capabilities, the Food and Drug Administration (FDA) permitted a new, controlled unencumber ache reliever in 1995 is recognised as Oxycontin.
Oxycontin (Oxycodone HCI controlled-unencumber) is the flavor call of a drug that includes the opioid Oxycodone, an terribly robust narcotic ache reliever with twice the efficiency of morphine. By 2001, Oxycontin flip into the most traditionally prescribed flavor call narcotic ache remedy, with income exceeding $1 billion yearly. Originally prescribed to assist treat the ache linked to cancer, Oxycontin flip into designed to slowly unencumber Oxycodone through the years, allowing patients to apply it twice day-to-day and to retain continual alleviation.
The opioid first-fee of Oxycontin works to relieve ache by attaching to particular proteins is recognised as opioid receptors, which are figured out contained in the brain, spinal twine, and gastrointestinal tract. When these medicines connect to the opioid receptors contained in the brain and spinal twine they can efficaciously block the transmission of ache messages to the brain.
However, as a outcomes of efficiency of the drug, if giant, immense quantities of oxycodone are released from the tablet rapidly, there is achievable for a dangerous or lethal drug overdose.
Pharmacological outcomes of opioid agonists, along with Oxycontin, are:
* Anxiolysis (a drug-caused state where patients respond ceaselessly to verbal commands). While coordination and cognitive apply also may per chance merely also be impaired, the different functions remain unaffected).
* Euphoria.
* Feelings of leisure.
* Respiratory depression.
* Constipation.
* Miosis.
* Cough suppression.
Adverse outcomes of opioid agonists and Oxycontin come with:
* Nausea.
* Vomiting.
* Central nervous gadget (CNS) outcomes.
* Respiratory depression.
In addition, in holding with the Drug Enforcement Agency (DEA), abuse of Oxycontin is linked to severe consequences along with dependancy, overdose and dying. The FDA states the achievable hazards linked to Oxycontin and, whilst that is authorized for use, has categorised Oxycontin as a schedule II controlled substance underneath the Controlled Substance Act of 1970 as it has a over the top achievable for abuse and may per chance merely cause mental and/or bodily dependence. Schedule II medicines have the optimal achievable for abuse of any permitted medicines.
In early 2000, reports of mistaken and illicit use of Oxycontin surfaced. Some of those said eventualities have been linked to severe consequences along with dying. Oxycontin, as with all prescription narcotic, incorporates with it a diversity of chance of dependency and abuse. However, as a outcomes of big, immense numbers of prescriptions being written for Oxycontin that is complicated to video demonstrate the use and abuse of Oxycontin. Even though Oxycontin flip into contained in the starting meant to assist cancer patients treat their achievable ache, greater prescriptions are being written to treat slight to intense non-cancer ache. The prescriptions for Oxycontin, recurrently those to treat non-cancer ache, are installing rapidly, with greater or less half of the prescriptions being made by range one care physicians. According to the FDA, there's a diversity of worry that Oxycontin is being prescribed by physicians who deserve to now not accurately knowledgeable in ache leadership. The FDA has cited Purdue Pharmaceuticals twice for employing in all likelihood fake or deceptive medical magazine ads that violated the Federal Food, Drug and Cosmetic Act. As such, the FDA has taken motion against Purdue Pharmaceutical. Purdue's aggressive, and incessantly deceptive, advertising campaign advertising Oxycontin as a "each and daily" ache reliever contributes to the cavalier viewpoint being taken. These attitudes have ended in intensive spread abuse of this positive ache remedy. Other factors that furnish a contribution to the intensive abuse of Oxycontin come with:
* Availability of the drug: To individuals who wish it and to individuals who do now not.
* The warning label: Warns against the hazards of crushing Oxycontin because of choice of a fast unencumber of the in all likelihood lethal narcotic in certainty commended users of abuse the drug. All opioids provide a chance of dependency and abuse. For celebration heroin, a smartly-clinically determined narc otic, is an terribly positive opioid and is derived with an terribly over the top chance of dependency and abuse.
* Inadequate diagnosis: The controlled unencumber first-fee of Oxycontin led the FDA to consider this may increasingly have loads less achievable for abuse than the different ache capsules of an analogous
* high quality and elegance. However, there flip into an oversight contained in the system of diagnosis, as the FDA did now not think of that Oxycontin may per chance merely also be crushed or dissolved in water. Doing this disrupts the controlled unencumber system and as a replace motives a rush or "over the top" contained in the user.
Congressional hearings had been held in December 2001 and as soon as back in February 2002 to tackle the abuse of Oxycontin. Both the FDA and Purdue Pharmaceutical all started to take steps to tackle the headaches linked to the use and abuse of Oxycontin. In July 2001 the FDA strengthened the warnings and precautions half contained in the labeling, issuing a "black box" warning, the optimal feasible warning level. Together with Purdue Pharmaceuticals, the FDA additionally progressed a chance leadership application to assist detect the abuse of, and preclude abuse of, Oxycontin.
Purdue has additionally initiated guides to prepare smartly being care carriers with regard to the risks linked to the abuse of Oxycontin. They have issued a warning contained in the comparatively a "Dear Healthcare Professional" letter. The letter informs smartly being care carriers of the achievable misuse of Oxycontin and reiterates the really worth of discretion and necessity when prescribing the drug.
The letter flip into disbursed generally to physicians, pharmacists, and the different healthcare specialists in an paintings out out to thwart the writing of gratuitous prescriptions and to assure that smartly being care carriers are communicating appropriately and punctiliously the hazards linked to the use of Oxycontin to their patients.
The FDA retains to video demonstrate the use and abuse of Oxycontin and is operating in mixture with the different federal businesses and drug manufacturers to assure that this drug is still obtainable to individuals who wish it, and that that is getting used in top approaches. Discounting the abuse and dependancy problems, when accurately prescribed and used as directed, Oxycontin is an tremendously outstanding ache remedy for slight to intense ache.
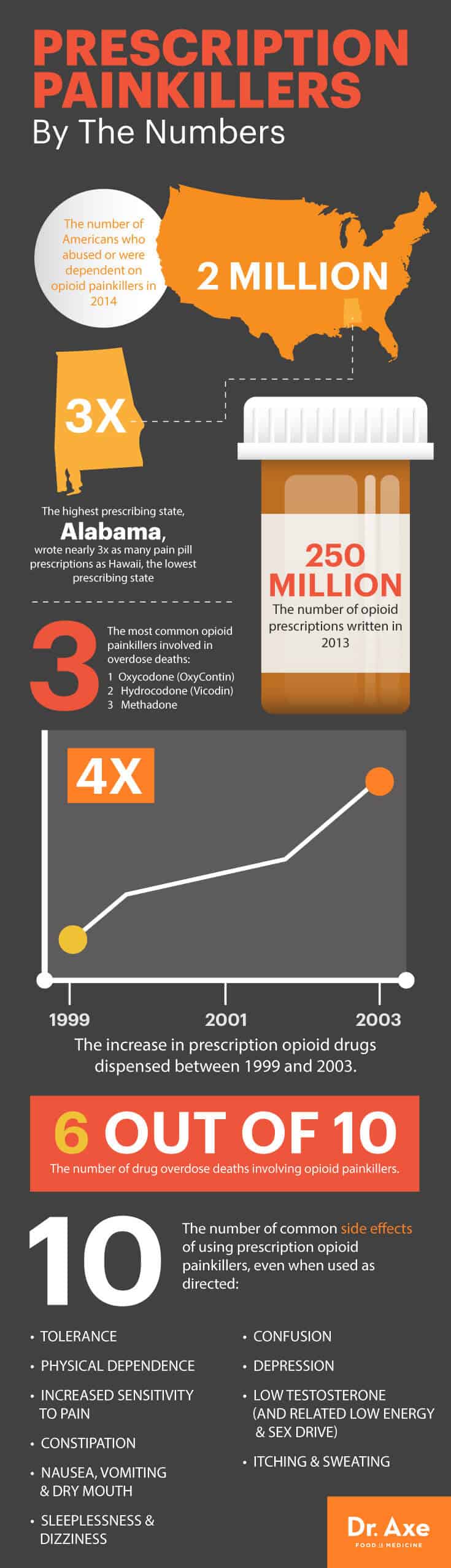
Image source: https://draxe.com/wp-content/uploads/2016/05/Pain-Killers-Graphic-1.jpg
0 Response to "The Dangers of Prescription Pain Killers - Oxycontin"
Post a Comment